From 2009 to current
<2025>
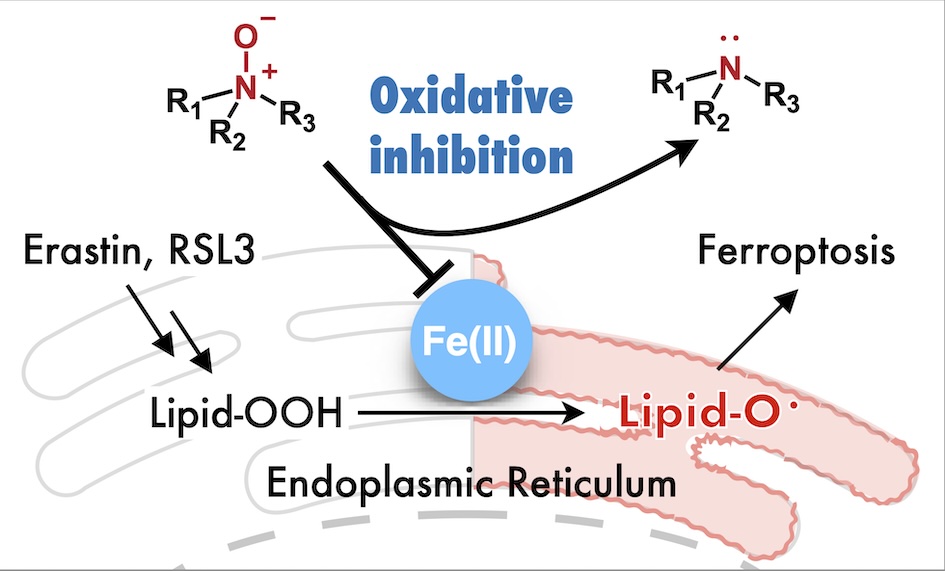
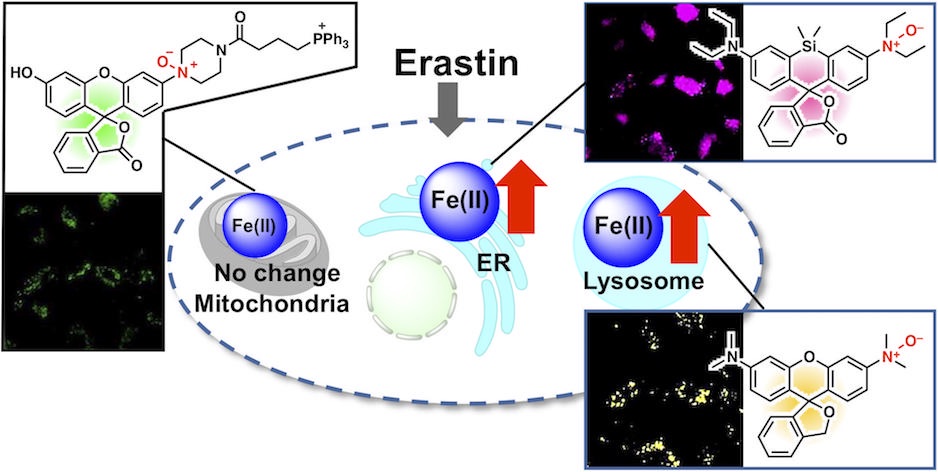
63. Inhibition of ferroptosis by N-oxide-based fluorescent probes via selective oxidation of ferrous ions [Open access]
Kanta Kawai, Rie Haruki, Shunsuke Nozawa, Hideko Nagasawa, Tasuku Hirayama*
Chem. Sci. 2025, 16, 11240. doi: 10.1039/d4sc07972h
[Selected as inside front cover]

62. Lysosomal lipid peroxidation contributes to ferroptosis induction via lysosomal membrane permeabilization [Open Access]
Yuma Saimoto, Daiki Kusakabe, Kazushi Morimoto, Yuta Matsuoka, Eisho Kozakura, Nao Kato, Kayoko Tsunematsu, Tomohiro Umeno, Tamiko Kiyotani, Shota Matsumoto, Mieko Tsuji, Tasuku Hirayama, Hideko Nagasawa, Koji Uchida, Satoru Karasawa, Mirinthorn Jutanom, Ken-ichi Yamada*
Nat. Commun. 2025, 16, 3554. doi: 10.1038/s41467-025-58909-w
<2024>
61. Design and synthesis of visible light-activatable photocaged peroxides for optical control of ROS-mediated cellular signaling
Mieko Tsuji,* Nobuyuki Koiso, Yufu Nishimura, Haruno Taira, Chinami Ogawa, Tasuku Hirayama,
Hideko Nagasawa*
Bioorg. Med. Chem. 2024, 111, 117863. doi: 10.1016/j.bmc.2024.117863
60. Application of fluorescent probe for labile heme quantification in physiological dynamics
Daisuke Tsuji, Tasuku Hirayama, Kanta Kawai, Hideko Nagasawa, Reiko Akagi*
Biochim. Biophys. Acta. 2024, 1868, 130707. doi: 10.1016/j.bbagen.2024.130707
59. Association of Poly(rC)-Binding Protein-2 with Sideroflexin-3 through TOM20 as an Iron Entry Pathway to Mitochondria
Danyang Mi, Izumi Yanatori, Hao Zheng, Yingyi Kong, Tasuku Hirayama, Shinya Toyokuni*
Free Radic. Res. 2024, 58, 261–275. doi: 10.1080/10715762.2024.2340711
<2023>
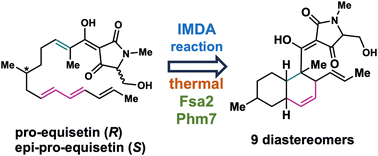
58. Elucidation of the stereocontrol mechanisms of the chemical and biosynthetic intramolecular Diels–Alder cycloaddition for the formation of bioactive decalins
Takumi Kariya, Hayato Hasegawa, Taro Udagawa, Yusaku Inada, Kyoko Nishiyama, Mieko Tsuji, Tasuku Hirayama, Tatsuo Suzutani, Naoki Kato, Shingo Nagano, Hideko Nagasawa*
RSC Adv. 2023, 13, 27828–27838. doi: 10.1039/D3RA04406H
57. Crucial Role of Iron in Epigenetic Rewriting during Adipocyte Differentiation Mediated by JMJD1A and TET2 Activity
Tomohiro Suzuki, Tetsuro Komatsu, Hiroshi Shibata, Akiko Tanioka, Diana Vargas, Reika Kawabata-Iwakawa, Fumihito Miura, Shinnosuke Masuda, Mayuko Hayashi, Kyoko Tanimura-Inagaki, Sumiyo Morita, Junki Kohmaru, Koji Adachi, Masayuki Tobo, Hideru Obinata, Tasuku Hirayama, Hiroshi Kimura, Juro Sakai, Hideko Nagasawa, Hideyuki Itabashi, Izuho Hatada, Takashi Ito, Takeshi Inagaki
Nuc. Acid Res. 2023, 51, 6120–6142. doi: 10.1093/nar/gkad342
56. Synthesis and Photochemical Properties of Caged Peroxides for Photocontrol of Cellular Oxidative Stress.
Mieko Tsuji,* Haruno Taira, Taro Udagawa, Tatsuya Aoki, Tasuku Hirayama, Hideko Nagasawa*
Chem. Commun. 2023, 59, 6706–6709. doi: 10.1039/D3CC01192E
<2022>
55. Irregular particle morphology and membrane rupture facilitate ion gradients in the lumen of phagosomes
Maksim V.Baranov, Melina Ioannidis, Sami Balahsioui, Auke Boersma, Rinsede Boer, Manoj Kumar, Masato Niwa, Tasuku Hirayama, Qintian Zhou, Terrence M. Hopkins, Pieter Grijpstra, Shashi Thutupalli, Stefano Sacanna, Geertvan den Bogaart*
Biophys. Rep. 2022, 2, 100069. doi: 10.1016/j.bpr.2022.100069
54. Haloperidol Prevents Oxytosis/Ferroptosis by Targeting Lysosomal Ferrous Ions in a Manner Independent of Dopamine D2 and Sigma-1 Receptors
Yoko Hirata,* Kohei Oka, Shotaro Yamamoto, Hiroki Watanabe, Kentaro Oh-hashi, Tasuku Hirayama, Hideko Nagasawa, Hiroshi Takemori, Kyoji Furuta*
ACS Chem. Neurosci. 2022, 13, 18, 2719–2727. doi:10.1021/acschemneuro.2c00398
53. Iron accelerates Fusobacterium nucleatum-induced CCL8 expression in macrophages and is associated with colorectal cancer progression
Taishi Yamane, Yohei Kanamori, Hiroshi Sawayama, Hiromu Yano, Akihiro Nita, Yudai Ohta, Hironori Hinokuma, Ayato Maeda, Akiko Iwai, Takashi Matsumoto, Mayuko Shimoda, Mayumi Niimura, Shingo Usuki, Noriko Yasuda-Yoshihara, Masato Niwa, Yoshifumi Baba, Takatsugu Ishimoto, Yoshihiro Komohara, Tomohiro Sawa, Tasuku Hirayama, Hideo Baba, Toshiro Moroishi*
JCI Insight, 2022, 7, e156802. doi: 10.1172/jci.insight.156802
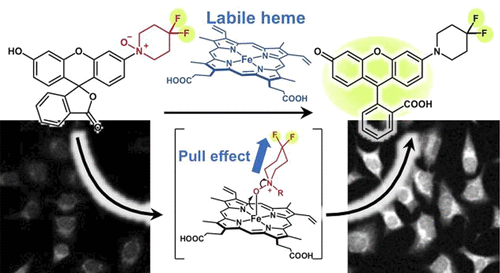
52. Molecular Imaging of Labile Heme in Living Cells Using a Small Molecule Fluorescent Probe
Kanta Kawai, Tasuku Hirayama,* Haruka Imai, Takanori Murakami, Masatoshi Inden, Isao Hozumi, Hideko Nagasawa
J. Am. Chem. Soc. 2022, 144, 9, 3793–3803. doi: 10.1021/jacs.1c08485 [Selected as Supplementary Cover Picture]
<2021>
51. Takayuki Sakai, Yoshiyuki Matsuo, Kensuke Okuda, Kiichi Hirota, Mieko Tsuji, Tasuku Hirayama & Hideko Nagasawa*
“Development of antitumor biguanides targeting energy metabolism and stress responses in the tumor microenvironment”
Sci. Rep. 2021, 11, 4852. doi: 10.1038/s41598-021-83708-w
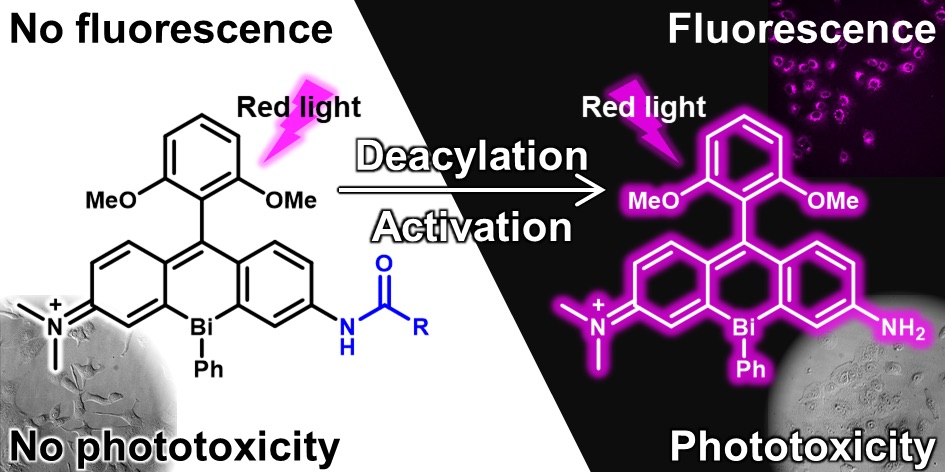
50. Akari Mukaimine, Tasuku Hirayama*, and Hideko Nagasawa
“Asymmetric bismuth-rhodamines as an activatable fluorogenic photosensitizer”
Org. Biomol. Chem. 2021, 19, 3611–3619, doi: 10.1039/D0OB02456B [Selected as Cover Picture]
<2020>
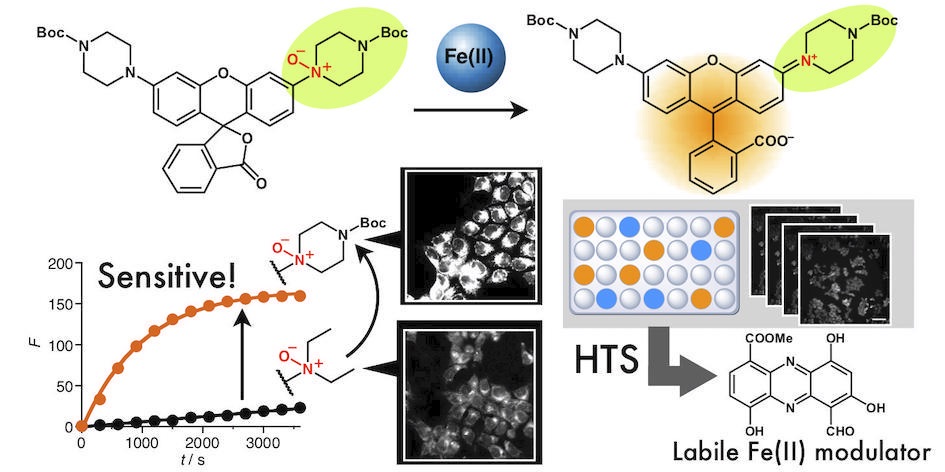
49. Tasuku Hirayama*, Masato Niwa, Shusaku Hirosawa, and Hideko Nagasawa
“High-Throughput Screening for the Discovery of Iron Homeostasis Modulators Using an Extremely Sensitive Fluorescent Probe”
ACS Sensors, 2020, 5, 2950–2958, doi: 10.1021/acssensors.0c01445
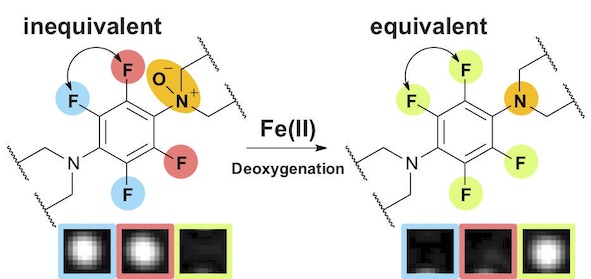
48. Ryo Kakiuchi, Tasuku Hirayama*, Daijiro Yanagisawa, Ikuo Tooyama, and Hideko Nagasawa
“A 19F-MRI probe for the detection of Fe(II) ions in an aqueous system”
Organic & Biomolecular Chemistry, 2020, 18, 5843–5849, doi: 10.1039/D0OB00903B
【Selected as the front cover picture of Org. Biomol. Chem. 2020, 18, Number 30】

47. Fumiya Ito, Izumi Yanatori, Yuki Maeda, Kenta Nimura, Satoki, Ito, Tasuku Hirayama, Hideko Nagasawa, Norihiko Kohyama, Yasumasa Okazaki, Shinya Akatsuka, Shinya Toyokuni*
“Asbestos conceives Fe(II)-dependent mutagenic stromal milieu through ceaseless macrophage ferroptosis and β-catenin induction in mesothelium”
Redox Biology, 2020, 36, 101616, doi: 10.1016/j.redox.2020.101616
46. Kota Koike, Masanobu Nagano, Masahiro Ebihara, Tasuku Hirayama, Mieko Tsuji, Hiroaki Suga, and Hideko Nagasawa*
“Design, Synthesis, and Conformation–Activity Study of Unnatural Bridged Bicyclic Depsipeptides as Highly Potent HIF-1 Inhibitors and Antitumor Agents”
Journal of Medicinal Chemistry, 2020, 63, 4022–4046, doi: 10.1021/acs.jmedchem.9b02039
<2019>
45. Zeling Xu, Pengchao Wang, Haibo Wang, Zuo Hang Yu, Ho Yu Au-Yeung, Tasuku Hirayama, Hongzhe Sun, and Aixin Yan*
“Zinc excess increases cellular demand for iron and decreases tolerance to copper in Escherichia coli“【open access】
Journal of Biological Chemistry, 2019, 294, 16978–16991. doi: 10.1074/jbc.RA119.010023
44. Takahiko Imai, Sena Iwata, Tasuku Hirayama, Hideko Nagasawa, Shinsuke Nakamura, Masamitsu Shimazawa, Hideaki Hara*
“Intracellular Fe2+ accumulation in endothelial cells and pericytes induces blood-brain barrier dysfunction in secondary brain injury after brain hemorrhage” 【open article】
Scientific Reports, 2019 , 9, 6228. doi: 10.1038/s41598-019-42370-z
43. Dan Cui, Mitsuru Arima, Tasuku Hirayama, Eiji Ikeda
“Hypoxia-induced disruption fo neural ascular barrier is mediated by the intracellular induction of Fe(II) ion”【open access】
Experimental Cell Research, 2019, 379, in press. doi: 10.1016/j.yexcr.2019.04.003
42. Aoi Isono, Mieko Tsuji, Yu Sanada, Akari Matsushita, Shinichiro Masunaga, Tasuku Hirayama, Hideko Nagasawa*
“Design, Synthesis, and Evaluation of Lipopeptide Conjugates of Mercaptoundecahydrododecaborate for Boron Neutron Capture Therapy”
ChemMedChem, 2019, 14, 823–832. doi: 10.1002/cmdc.201800793
【Selected as the back cover picture of ChemMedChem 2019, issue 8】doi/10.1002/cmdc.201900213 (Designed by Dr. Tsuji)

41. Tasuku Hirayama*
[review] Fluorescent probes for the detection of catalytic Fe(II) ion
Free Radical and Biology and Medicine, 2019, 133, 38–45. doi: 10.1016/j.freeradbiomed.2018.07.004
40. Tasuku Hirayama*, Masatoshi Inden*, Hitomi Tsuboi, Masato Niwa, Yasuhiro Uchida, Yuki Naka, Isao Hozumi, Hideko Nagasawa
“A Golgi-targeting fluorescent probe for labile Fe(II) to reveal abnormal cellular iron distribution induced by dysfunction of VPS35” 【open article】
Chemical Science, 2019, 10, 1514–1521. doi: 10.1039/C8SC04386H.
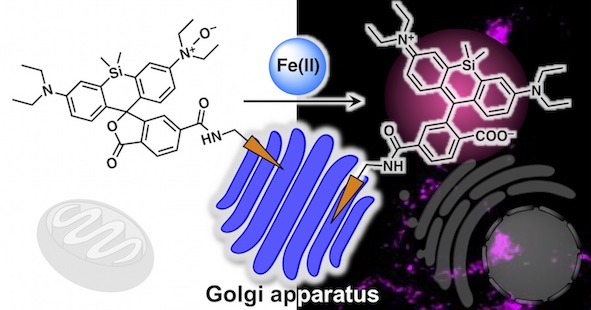
39. Tasuku Hirayama*, Ayaji Miki, Hideko Nagasawa
“Organelle-specific analysis of labile Fe(II) during ferroptosis by using a cocktail of various colour organelle-targeted fluorescent probes”
Metallomics, 2019, 11, 111–117, doi: 10.1039/C8MT00212F.
【Selected as the front cover of Metallomics 2019, issue 1!】(Designed by T. Hirayama)

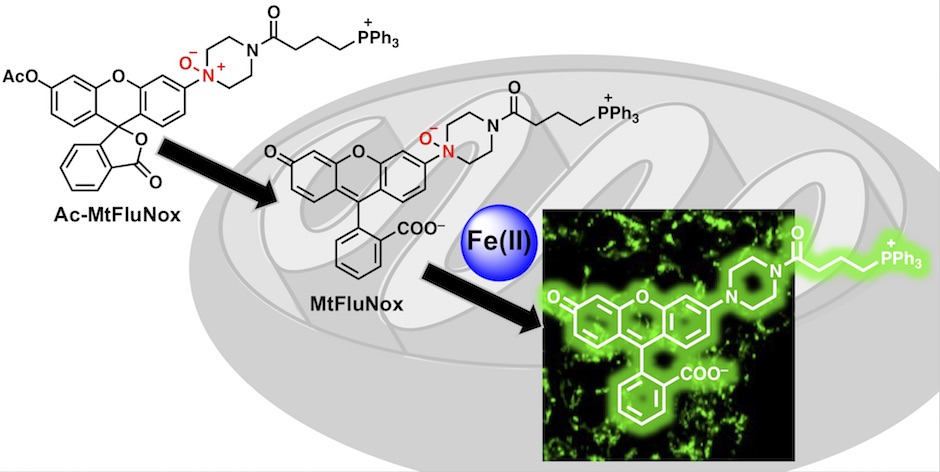
<2018>
38. Tasuku Hirayama*, Satoki Kadota, Masato Niwa, Hideko Nagasawa
“A mitochondria-targeted fluorescent probe for selective detection of mitochondrial labile Fe(II)”
Metallomics, 2018, 10, 794–801, doi: 10.1039/C8MT00049B.
【Selected as the front cover picture of Metallomics 2018, issue 6!】(Designed by T. Hirayama)

37. Kenji Sakamoto*, Taishi Suzuki, Kosuke Takahashi, Takumi Koguchi, Tasuku Hirayama, Asami Mori, Tsutomu Nakahara, Hideko Nagasawa, Kunio Ishii
“Iron-chelating agents attenuate NMDA-Induced neuronal injury via reduction of oxidative stress in the rat retina”
Exp. Eye Res. 2018, 171, 30–36. doi: 10.1016/j.exer.2018.03.008
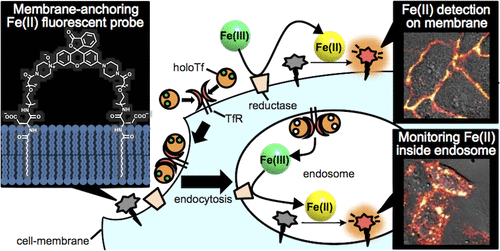
36. Masato Niwa, Tasuku Hirayama*, Ikumi Oomoto, Dan Ohtan Wang, Hideko Nagasawa
“Fe(II) ion release during endocytotic uptake of iron visualized by a membrane-anchoring Fe(II) fluorescent probe”
ACS Chem. Biol.,13, 1853–1861, doi:10.1021/acschembio.7b00939
【Selected as “ACS Editor’s Choice”!】
【Top 20 most downloaded article in May–June 2018!】
<2017>

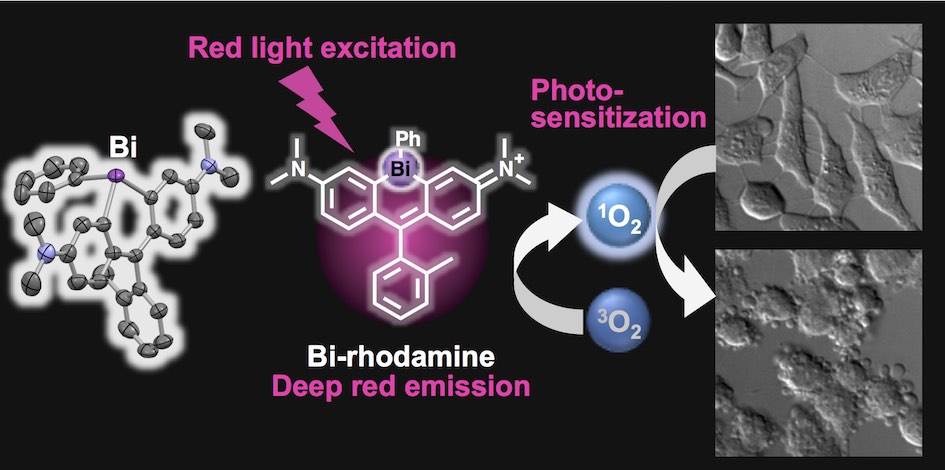
35. Tasuku Hirayama*, Akari Mukaimine, Kenta Nishigaki, Hitomi Tsuboi, Shusaku Hirosawa, Kensuke Okuda, Masahiro Ebihara, Hideko Nagasawa*
“Bismuth-rhodamine: a new red light-excitable photosensitizer”
Dalton Trans. 2017, 46, 15991–15995. doi: 10.1039/c7dt03194g
【Selected as the back cover picture!】Back cover (designed by A. Mukaimine) : Dalton Trans., 2017,46, 16328
34. Yasumasa Ikeda, Yuya Horinouchi, Hirofumi Hamano, Tasuku Hirayama, Seiji Kishi, Yuki Izawa-Ishizawa, Masaki Imanishi, Yoshito Zamami, Kenshi Takechi, Licht Miyamoto, Keisuke Ishizawa, Ken-ichi Aihara, Hideko Nagasawa, Koichiro Tsuchiya, Toshiaki Tamaki*
“Dietary iron restriction alleviates renal tubulointerstitial injury induced by protein overload in mice”
Sci. Rep. 2017, 7, 10621. doi: 10.1038/s41598-017-11089-0【open article】
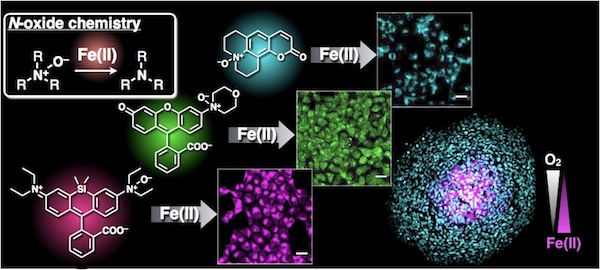
33. Tasuku Hirayama,* Hitomi Tsuboi, Masato Niwa, Ayaji Miki, Satoki Kadota, Yukie Ikeshita, Kensuke Okuda, Hideko Nagasawa*
“A universal fluorogenic switch for Fe(II) ion based on N-oxide chemistry permits the visualization of intracellular redox equilibrium shift towards labile iron in hypoxic tumor cells”
Chem. Sci. 2017, 8, 4858–4866. doi: 10.1039/C6SC05457A【open article】
32. Keisuke Oshima, Yasumasa Ikeda, Yuya Horinouchi, Hiroaki Watanabe, Hirofumi Hamano, Yoshitaka Kihira, Seiji Kishi, Yuki Izawa-Ishizawa, Licht Miyamoto, Tasuku Hirayama, Hideko Nagasawa, Keisuke Ishizawa, Koichiro Tsuchiya, Toshiaki Tamaki*
“Iron suppresses erythropoietin expression via oxidative stress-dependent hypoxia-inducible factor-2 alpha inactivation”
Lab. Invest. 2017, 97, 555–566. doi: 10.1038/labinvest.2017.11
31. Tasuku Hirayama*, Hideko Nagasawa
[review] “Chemical tools for detection of Fe ions
J. Clin. Biochem. Nut. 2017, 60, 39–48.
<2016>
30. 平山 祐
【紹介記事】「Click-EM—生体分子を電子顕微鏡で観察する新しい手法」
News & Hot Paper Digest、実験医学、2016、Vol. 34、No.19(12月号)、3170–3171
29. 平山 祐
【総説】「蛍光プローブ分子を使った生細胞での高感度二価鉄検出」
化学工業第67巻、p.41–47、2016年8月
28. Fumiya Ito, Takahiro Nishiyama, Lei Shi, Masahiko Mori, Tasuku Hirayama, Hideko Nagasawa, Hiroyuki Yasui, Shinya Toyokuni*
“Contrasting intro-and extracellular distribution of catalytic ferrous iron in ovalbumin-induced peritonitis”
Biochem. Biophys. Res. Commun. 2016, 476, 600–606.
27. Yue Wang, Yasumasa Okazaki, Lei Shi, Hiro Kohda, Minoru Tanaka, Kentaro Taki, Tomoki Nishioka, Tasuku Hirayama, Hideko Nagasawa, Yoriko Yamashita, Shinya Toyokuni*
“Role of hemoglobin and transferrin in multi-wall carbon nanotube-induced mesothelial injury and carcinogenesis”
Cancer Sci. 2016, 107, 250-257
26.Kozo Hattori, Kota Koike, Kensuke Okuda, Tasuku Hirayama, Masahiro Ebihara, Mei Takenaka, Hideko Nagasawa*
“Solution-Phase Synthesis and Biological Evaluation of Triostin A and its Analogues”
Org. Biomol. Chem. 2016,14, 2090-2111
<2015>
25. Masahiko Mori, Fumiya Ito, Lei Shi, Yue Wang, Chiharu Ishida, Yuka Hattori, Masato Niwa, Tasuku Hirayama, Hideko Nagasawa, Akira Iwase, Fumitaka Kikkawa, Shinya Toyokuni*
“Ovarian endometriosis-associated stromal cells reveal persistently high affinity for iron”
Redox Bioloby, 2015, 6, 578–586.
24. Takahiro Hikiji, Junpei Norisada, Yoko Hirata, Kensuke Okuda, Hideko Nagasawa, Yusuke Ishigaki, Gen Sobue, Kazutoshi Kiuchi, Kentaro Oh-hashi*
“A highly sensitive and quantitative assay of IRE1 activity using the small luciferase NanoLuc: evaluation of ALS-related genetic and pathological factors”
Biochem. Biophys. Res. Commun., 2015, 463, 881–887
23. 平山祐、丹羽正人、奥田健介、永澤秀子
【総説】「N-oxideの化学を利用した鉄(II)イオン蛍光プローブ分子の開発」
Chemical Biology, 2015, 8, 9–11.
22. Hitoshi Tsugawa, Hideki Mori, Juntaro Matsuzaki, Tatsuhiro Masaoka, Tasuku Hirayama, Hideko Nagasawa, Yasubumi Sakakibara, Makoto Suematsu, and Hidekazu Suzuki*
“Nordihydroguaiaretic Acid Disrupts the Antioxidant Ability of Helicobacter pylori through the Repression of SodB Activity In Vitro”
Biomed. Res. International, 2015 doi:10.1155/2015/734548
21. Noriko Nozawa-Suzuki, Hideko Nagasawa, Ken Ohnishi, Ken-ichirou Morishige*
“The inhibitory effect of hypoxic cytotoxin on the expansion of cancer stem cells in ovarian cancer”
Biochem. Biophys. Res. Commun., 2015, 457, 706–711
20. Yuka Hattori, Takahiro Mukaide, Li Jiang, Tomomi Kotani, Hiroyuki Tsuda, Yukio Mano, Seiji Sumigawa, Tasuku Hirayama, Hideko Nagasawa, Fumitaka Kikkawa, and Shinya Toyokuni*
“Catalytic ferrous ion in amniotic fluid as a predictive marker of human maternal-fetal disorders”
J. Clin. Biochem. Nut., 2015, 56, 57-63
<2014>
19. 奥田健介、平山祐、永澤秀子
【総説】「低酸素・低栄養環境関連分子を標的とする創薬研究の現状」
放射線生物研究, 2015, 49(4), 341–357.
18. Masato Niwa, Tasuku Hirayama*, Kensuke Okuda Hideko Nagasawa*
“A new class of high-contrast Fe(II) selective fluorescent probes based on spirocyclized scaffolds for visualization of intracellular labile iron delivered by transferrin”
Org. Biomol. Chem., 2014, 12, 6535-6746 (selected as front cover!)

17. Kosuke Narise, Kensuke Okuda, Yukihiro Enomoto, Tasuku Hirayama, Hideko Nagasawa*
“Optimization of biguanide derivatives as selective antitumor agents blocking adaptive stress responses in the tumor microenvironment”
Drug Design, Development and Therapy, 2014, 2014(8), 701–717
16. Tasuku Hirayama, Satoshi Ueda, Takahiro Okada, Norihiko Tsurue, Kensuke Okuda and Hideko Nagasawa*
“Facile One-Pot Synthesis of [1, 2, 3]Triazolo[1, 5-a]Pyridines from 2-Acylpyridines by Copper(II)-Catalyzed Oxidative N-N Bond Formation”
Chemistry – A European Journal, 2014, 20, 4156-4162

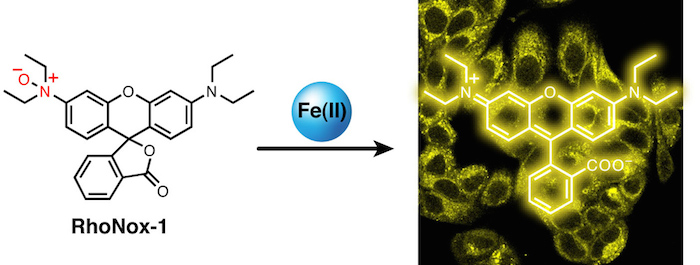
15. Takahiro Mukaide, Yuka Hattori, Nobuaki Misawa, Satomi Funahashi, Li Jiang, Tasuku Hirayama, Hideko Nagasawa and Shinya Toyokuni*
“Histological detection of catalytic ferrous iron with the selective turn-on fluorescent probe RhoNox-1 in a Fenton reaction-based rat renal carcinogenesis model”
Free Radical Research, 2014, 48, 990-995
<2013>
14. Mieko Tsuji, Satoshi Ueda, Tasuku Hirayama, Kensuke Okuda, Yoshiaki Sakaguchi, Aoi Isono, Hideko Nagasawa*
“FRET-based imaging of transbilayer movement of pepducin in living cells by novel intracellular bioreductively activatable fluorescent probes”
Organic & Biomolecular Chemistry, 2013, 11, 3030-3037

13. Tasuku Hirayama, Kensuke Okuda, Hideko Nagasawa*
“A highly selective turn-on fluorescent probe for iron(II) to visualize labile iron in living cells”
Chemical Sciences, 2013, 4, 1250-1256
<2012>
12. Kensuke, Okuda, Yasuyuki Okabe, Tetsuya Kadonosono, Takahiro Ueno, Bahaa Gamal Mohamed Youssif, Shinae Kondoh-Kizaka, Hideko Nagasawa
“2-Nitroimidazole-Tricarbocyanine Conjugate as a Near-Infrared Fluorescent Probe for in Vivo Imaging of Tumor Hypoxia.”
Bioconjugate Chemistry, 2012, 23, 324-329.
11. Hitoshi Miyakoshi, Seiji Miyahara, Tatsushi Yokogawa, Khoon Tee Chong, Junko Taguchi, Kanji Endoh, Wakako Yano, Takeshi Wakasa, Hiroyuki Ueno, Yayoi Takao, Makoto Nomura, Satoshi Shuto, Hideko Nagasawa, Masayoshi Fukuoka
“Synthesis and discovery of N-carbonylpyrrolidine- or N-sulfonylpyrrolidine-containing uracil derivatives as potent human deoxyuridine triphosphatase inhibitors.”
Journal of Medicinal Chemistry, 2012, 55, 2960-2969.
10. Bahaa Gamal Mohamed Youssif, Kensuke Okuda, Tetsuya Kadonosono, Ola Ibrahim Abdel Razek Salem, Alaa Arafat Mohamed Hayallah, Mostafa Ahmed Hussein, Shinae Kizaka-Kondoh, Hideko Nagasawa
“Development of a hypoxia-selective near-infrared fluorescent probe for non-invasive tumor imaging.”
Chemical Pharmaceutical Bulletin, 2012, 60, 402-407.
9. Tasuku Hirayama, Masayasu Taki, Kazushi Akaoka, Yukio Yamamoto
“Development of a dual functional luminescent sensor for zinc ion based on a peptidic architecture.”
Bioorganic & Medicinal Chemistry Letters, 2012, 22, 7410-7413.
<2011>
8. Hideko Nagasawa
“Pathophysiological Response to Hypoxia — From the Molecular Mechanisms of Malady to Drug Discovery:Drug Discovery for Targeting the Tumor Microenvironment”
Journal of Pharmacological Sciences, 2011, 115, 446-452.
7. Sadaaki Kimura, Shin-ichiro Masunaga, Tomohiro Harada, Yasuo Kawamura, Satoshi Ueda, Kensuke Okuda, Hideko Nagasawa
“Synthesis and evaluation of cyclic RGD-boron cluster conjugates to develop tumor-selective boron carriers for boron neutron capture therapy”
Bioorganic & Medicinal Chemistry, 2011, 19, 1721-1728.
6. Hisanori Hattori, Kensuke Okuda, Tetsuji Muirase, Yuki Shigetsura, Kousuke Narise, Gregg L. Semenza, Hideko Nagasawa
“Isolation, identification, and biological evaluation of HIF-1-modulating compounds from Brazilian green propolis”
Bioorganic & Medicinal Chemistry, 2011, 19, 5392-5401.
<2010>
5. Satoshi Ueda, Takahiro Okada, Hideko Nagasawa
“Oxindole synthesis by palladium-catalysed aromatic C—H alkenylation”
Chemical Communications, 2010, 2462-2464.
<2009>
4. Kensuke Okuda, Takashi Hirota, David A Kingery, Hideko Nagasawa
“Synthesis of a fluorine-substituted puromycin derivative for Brønsted studies of ribosomal-catalyzed peptide bond formation.”
The Journal of Organic Chemistry, 2009, 74, 2609-2612.
3. Shinae Kizaka-Kondoh, Hideko Nagasawa
“Significance of nitroimidazole compounds and hypoxia-induceble factor-1 for imaging tumor hypoxia”
Cancer Science, 2009, 100, 1366-1373.
2. Satoshi Ueda, Hideko Nagasawa
“Copper-catalyzed synthesis of benzoxazoles via a regioselective C-H functionalization/C-O bond formation under an air atmosphere.”
The Journal of Organic Chemistry, 2009, 74, 4272-7277
1. Satoshi Ueda, Hideko Nagasawa
“Facile synthesis of 1,2,4-triazoles via a copper-catalyzed tandem addition-oxidative cyclization.”
Journal of the American Chemical Society, 2009, 131, 15080-15081